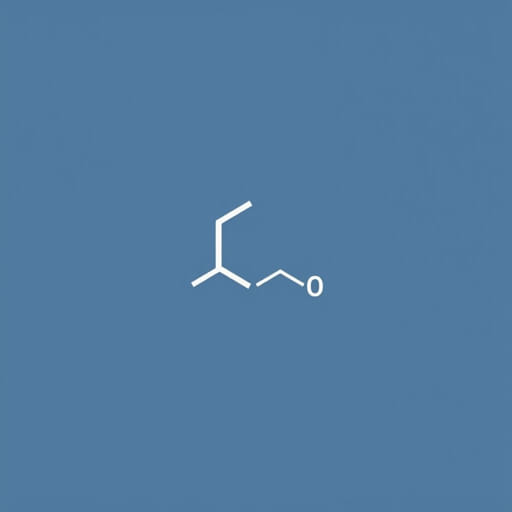
The chemistry of esters is a fascinating field that bridges the gap between organic chemistry and practical applications in everyday life. Among these compounds, esters of formic acid hold a special place due to their unique chemical structure, reactivity, and versatility. Formic acid, also known as methanoic acid, is the simplest carboxylic acid with the chemical formula HCOOH. When it reacts with alcohols, it forms esters, which are widely used in fragrances, flavorings, and industrial processes. Understanding the formation, properties, and applications of formic acid esters is crucial for chemists and enthusiasts who wish to explore both theoretical and practical aspects of organic chemistry.
Formation of Esters from Formic Acid
The process of esterification is central to producing esters of formic acid. Esterification is a chemical reaction between an alcohol and a carboxylic acid, typically catalyzed by an acid such as sulfuric acid. In the case of formic acid, the reaction can be represented as
HCOOH + R-OH → HCOOR + H2O
Here, R-OH represents the alcohol, and HCOOR is the resulting ester. The reaction is reversible, and water is produced as a byproduct. To maximize ester formation, techniques such as using excess alcohol or removing water during the reaction are commonly employed.
Mechanism of Esterification
The mechanism of ester formation from formic acid involves several steps
- Protonation of the carbonyl group of formic acid increases its electrophilicity.
- Nucleophilic attack by the alcohol’s hydroxyl group occurs on the carbonyl carbon.
- Formation of a tetrahedral intermediate stabilizes the system temporarily.
- Loss of water and deprotonation results in the formation of the ester.
This mechanism explains why acid catalysts are important in esterification, as they help activate the formic acid molecule and speed up the reaction.
Physical Properties of Formic Acid Esters
Formic acid esters exhibit a range of physical properties depending on the alcohol component used in their synthesis. Generally, they are characterized by their distinct fragrances and volatility, making them valuable in perfumery and flavor industries. Some common examples include methyl formate and ethyl formate. Methyl formate has a fruity smell and is often used as a solvent or in the production of pharmaceuticals. Ethyl formate, with a similar fruity aroma, is commonly found in flavoring agents and is also used as a fumigant in food processing.
Boiling and Solubility
Esters of formic acid generally have lower boiling points than their corresponding alcohols due to the absence of hydrogen bonding between ester molecules. They are moderately soluble in water because the formyl group can form hydrogen bonds with water molecules. Solubility decreases as the hydrocarbon chain of the alcohol increases. For instance, methyl formate is highly soluble in water, while butyl formate has limited solubility.
Chemical Properties
Formic acid esters are reactive compounds due to the presence of the ester functional group. Some of their chemical properties include
- Hydrolysis Esters can be hydrolyzed back to formic acid and alcohol in the presence of an acid or base. Acidic hydrolysis is slower but reversible, while basic hydrolysis (saponification) is faster and yields a formate salt.
- Reduction Esters can be reduced to primary alcohols using reducing agents such as lithium aluminum hydride (LiAlH4).
- Transesterification Esters can undergo exchange reactions with other alcohols to form new esters, which is widely used in industrial synthesis.
Stability and Reactivity
Although esters of formic acid are relatively stable under normal conditions, they can be sensitive to heat and strong acids or bases. Overheating or prolonged exposure to strong acids may lead to decomposition. In industrial applications, maintaining appropriate conditions ensures that the esters remain intact while allowing their desired reactions to occur efficiently.
Applications of Formic Acid Esters
Formic acid esters find a variety of applications in both industrial and consumer contexts. One of the primary uses is in the flavor and fragrance industries. Methyl and ethyl formates are commonly added to foods, beverages, and perfumes to impart fruity and pleasant aromas. Their volatility and safety in small quantities make them ideal for such purposes.
Industrial Uses
In addition to flavoring, formic acid esters are used as intermediates in chemical synthesis. They can serve as solvents for cellulose acetate, resins, and other organic compounds. Some esters are also used in the production of pharmaceuticals and agrochemicals. Their reactivity allows chemists to transform them into a wide range of valuable compounds efficiently.
Environmental and Safety Considerations
While generally safe in controlled applications, large quantities of formic acid esters can be flammable and may pose inhalation risks. Proper storage and handling are essential, particularly in industrial settings. Safety measures such as adequate ventilation, use of protective equipment, and fire prevention protocols are crucial to minimize risks.
Examples of Common Formic Acid Esters
- Methyl FormateClear, colorless liquid with a fruity odor, used as a solvent and in the manufacture of formamide.
- Ethyl FormateVolatile ester with a sweet smell, commonly used in flavoring and fumigation.
- Propyl FormateLess common, used in perfumery for fruity and floral scents.
- Butyl FormateOften utilized in the production of synthetic flavors and fragrances.
Esters of formic acid play an important role in both chemistry and industry. Their formation through esterification, unique physical and chemical properties, and versatile applications make them a valuable class of compounds. Understanding how these esters behave, from reactivity to solubility and aroma, allows chemists and industry professionals to utilize them effectively. From enhancing flavors in foods to serving as intermediates in complex syntheses, formic acid esters illustrate the importance of simple organic compounds in everyday life and industrial processes. Their study offers insight into the broader field of organic chemistry while providing practical benefits that impact numerous sectors.